 |

Home

Course
Information
WarmUp Exercise
Homework
Syllabus
Bulletin Board
|
|
Physics 416/517:
Problem Set #7
Due by 5 PM Friday,
April 11th, 2003
Reif, Problems 7.1, 7.3, 7.5, 7.7,
7.14,* 7.20
Problem 7:*
The energy levels of a particle trapped
in a cubical box of volume V = L3 are given by
En = (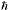 2 2 2/2mL2)(nx
2+ny2+nz2).
We can use this to model a single atom of helium trapped
in a metal crystal. Assume the "box" has sides of length
L = 1 angstrom (10-8 cm). 2/2mL2)(nx
2+ny2+nz2).
We can use this to model a single atom of helium trapped
in a metal crystal. Assume the "box" has sides of length
L = 1 angstrom (10-8 cm).
- Write down an expression
for the partition function, do not attempt to evaluate
the series.
- For T = 300 K, evaluate the first
few terms of the partition function
- Approximate the probability of
finding the atom in its first excited
state.
- How big would the box have to be to
have a probability near 0.1 of finding the atom in the
first excited state?
Hint: you may want to try setting this up
in Excel, Matlab, Mathematica, or any other numerical
tool that you like.
Note: * indicates
problems for 517 and 416 honors students
only
|




