
Home

WarmUp

What is Chemistry Good For?

Puzzle
Course Information

Communications
|
 |
 "What is Chemistry Good For?"
"What is Chemistry Good For?"
The for-credit questions are available at the end of this page.
Please respond before 5 AM, Monday, April 23th, 2001.

Chemistry is Good for Making Materials
The Stoichiometry of Carpet Formation
If you want things, you have to build them. In ancient times, people used what was readily
available around them, like rocks, trees and mud. Gradually over the years, humans
have gotten increasingly adept at taking control of their environment and squeezing new,
better materials with which to build things.
Once when I was camping with a bunch of other chemists, we met a group of campers from
Northern California. After a few friendly words about the weather and the
beauty of the Sierra Mountains, they asked us what we did for a living. Shockingly,
when we said that we were chemists, they flat out told us that we were NOT WELCOME.
They seriously did not want to associate with the types of people who were polluting the earth.

 Once our shock subsided, we asked them what their tent was made of? They had a very high quality nylon tent that they were very proud of.
We asked them how they purify their water. Like many other hikers, they carried a membrane filtration system and had iodine tablets as a back up.
After we asked them what their pants were made from, they got the picture.
Gore-tex is not a natural fiber either. These chemist-hating campers were
depending on evil chemists to provide most of their supplies and equipment.
Because of the work of chemists and chemical engineers,
these hikers had tastier food and lighter, more durable equipment.
Once our shock subsided, we asked them what their tent was made of? They had a very high quality nylon tent that they were very proud of.
We asked them how they purify their water. Like many other hikers, they carried a membrane filtration system and had iodine tablets as a back up.
After we asked them what their pants were made from, they got the picture.
Gore-tex is not a natural fiber either. These chemist-hating campers were
depending on evil chemists to provide most of their supplies and equipment.
Because of the work of chemists and chemical engineers,
these hikers had tastier food and lighter, more durable equipment.

So what does all this have to do with carpet?
I used to think that all carpet was either wool or cotton. Most is not! What is carpet made from?
Where does it come from? How much is bought and sold each year in the US? Right now, the most
common carpet is made from nylon fibers, which are durable and cheap. The annual world production
of nylon is 2800 million pounds. Yes, 2800 million pounds! Since nylon is not a natural substance,
it must be synthesized from somewhere. Because of limitations posed by the law of conservation of
mass, if you need to make 2800 million pounds of nylon, you need to start with at least 2800 million
pounds of something else. That something else is petroleum oil, which was originally pumped out of
the ground.

Starting from the end and working forward, here is the process. Nylon chips are melted and forced
through holes in a metal disk called a spinneret. The rate at which the nylon is forced through
the spinneret and drawn out controls the diameter of the fibers. Prior to the spinning of the
fibers, nylon is obtained as a tough ivory-like substance from the reaction of adipic acid and
hexamethylene diamine. Both of these chemicals have functional groups (sites of potential
reactivity) on both ends. Wallace Carothers of DuPont discovered that the reaction of these
two molecules resulted in very long molecules. The amine groups (NH2) on hexamethylene diamine
react with the acid groups (COOH) on the adipic acid just like the C terminus of an amino acid
reacts with the N terminus of an amino acid to form the peptide bonds of proteins.
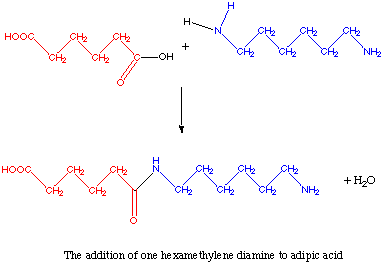
A single reaction between adipic acid and hexamethylene diamine is shown below.
It is possible to add another adipic acid to the end of every hexamethylene diamine and to
add another hexamethylene to every adipic acid. In this way, infinitely long molecular
chains are possible. To insure that good nylon is formed, the water byproduct must be
removed from the reaction.
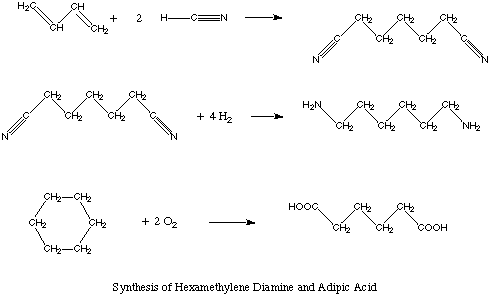
In the industrial nations, 2620 million pounds of hexamethylene diamine are produced each year.
It is synthesized by a variety of routes, one being the hydrocyanation of butadiene to form
adiponitrile. This process uses extremely toxic hydrogen cyanide gas. To minimize risks
associated with the storage and handling of hydrogen cyanide, engineers have developed a
process that uses the hydrogen cyanide immediately after it is generated.
And here are a few good links to get you started.
1. 2.
2. 3.
3. 4.
4.
This site is made possible by funding from the National Science Foundation (DUE-9981111).
|




