 |
 "What is Chemistry Good For?"
"What is Chemistry Good For?"
The for-credit questions are available at the end of this page.
Please respond before 5 AM, Monday, October 16th, 2000.
Original text and research by Ryan White, an IUPUI Undergraduate Student as part of a C105 Honors Project.

Chemistry is Good for Curing Cancer
Boron Neutron Capture Therapy
The fifth element in the periodic table, boron, is receiving quite a bit of attention in
the world of medicine for the treatment of tumors. Boron is an element that can be
irradiated with neutrons to release high-energy alpha particles and gamma rays
that can destroy tumor cells. How does this work?
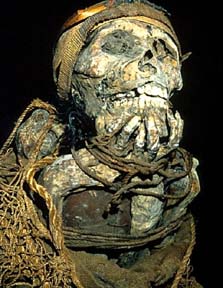
Lets start with a brief history lesson on boron. Boron has been used as far back in history
as early Egypt. Mummification was a regular practice for the burial of the deceased.
Mummification depended upon an ore known as natron, which contained borates as well as
some other common salts. Natron was a highly prized substance and was very expensive.
Boron was also used for welding in China and glassmaking in ancient Rome.
Boron minerals were discovered in Southern California about 1869. Currently about
one-half the mined borax used each year in the world comes from U.S. Borax in California.
They produce 500,000 metric tons or 1,100,000,000 pounds. That is a large
amount of borax mining.
 We have learned that boron likes to combine with oxygen to form several different compounds.
We also know that one element over from boron is carbon which, like boron, also likes to
combine with oxygen to form compounds such as carbon dioxide. However, carbon also likes to
combine with hydrogen to form hydrocarbons for fuels such as gasoline. You might expect
boron to also combine with hydrogen, but in nature there is no known combination of
boron and hydrogen. In 1910, Alfred Stock, began working on combining boron and hydrogen
to make boron-hydrides and he succeeded. However, these compounds were very expensive
and very reactive. It was not until the U.S. Army began research on boron-hydrides as a
possible rocket fuel that this research proved to be useful. This research
continued until 1948 when the research facility was washed out with carbon tetrachloride,
not realizing that carbon tetrachloride and decaborane form an explosive mixture similar
to nitroglycerine and the plant was blown away. Current stockpiles of pentaborane
(B5H9) and decaborane (B10H18) are being destroyed because these compounds are
powerful central nervous system toxins.
We have learned that boron likes to combine with oxygen to form several different compounds.
We also know that one element over from boron is carbon which, like boron, also likes to
combine with oxygen to form compounds such as carbon dioxide. However, carbon also likes to
combine with hydrogen to form hydrocarbons for fuels such as gasoline. You might expect
boron to also combine with hydrogen, but in nature there is no known combination of
boron and hydrogen. In 1910, Alfred Stock, began working on combining boron and hydrogen
to make boron-hydrides and he succeeded. However, these compounds were very expensive
and very reactive. It was not until the U.S. Army began research on boron-hydrides as a
possible rocket fuel that this research proved to be useful. This research
continued until 1948 when the research facility was washed out with carbon tetrachloride,
not realizing that carbon tetrachloride and decaborane form an explosive mixture similar
to nitroglycerine and the plant was blown away. Current stockpiles of pentaborane
(B5H9) and decaborane (B10H18) are being destroyed because these compounds are
powerful central nervous system toxins.

Other spinoffs of this initial research include the Nobel Prize winning work of H. C. Brown
of Purdue University. He used diborane and other boron hydrides to perform extremely
useful transformations on organic compounds. The addition of boron hydrides to
certain organic compounds (hydroboration) allows for the efficient synthesis of many
specialty chemicals including pharmaceuticals.
Now that we have a good history on boron let us see how it is being used today for the
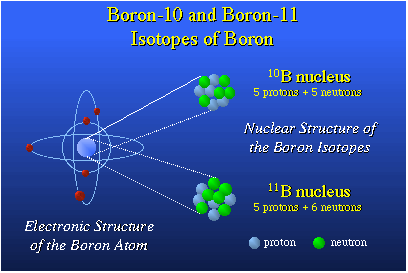
treatment of tumors. Boron has two isotopes, boron-10 and boron-11. Boron-10 is the
only light element with an extremely high propensity to bind with a slow moving neutron.
These slow neutrons are referred to as thermal neutrons. When boron-10 picks up this
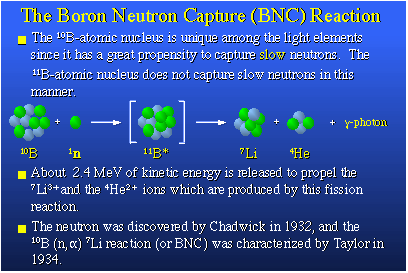
thermal neutron it becomes a high-energy boron-11. This boron-11 nucleus is quite unstable,
so it fissions or blows up releasing two heavy particles, a lithium-7 nucleus and a helium-4
nucleus plus a gamma photon. The heavy lithium-7 and helium-4 nuclei exit at a very high
speed making them very deadly to the cell they originated in. If researchers could
selectively incorporate boron-10 into cancer cells, they could then irradiate the patient
with thermal neutrons. The destruction of tissue would be localized to only the cancerous
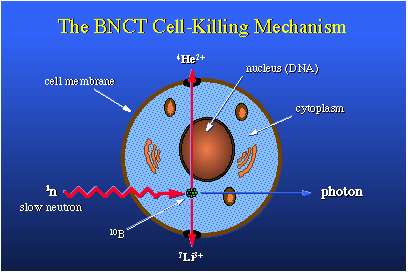
cells, since they are the only ones that contain boron-10. The BNC (boron neutron capture)
would kill the targeted tumor cells and the body could heal itself, replacing the dead tumor
tissue with normal tissue. The attractive part of this treatment is that the length of
travel of the lithium and helium ions produced from BNC is only about one cell thick.
Due to their weight and energy, they virtually tear the cell to pieces, but the damage ends there.
This therapy is being studied as a treatment for glioblastoma multiform, a very deadly brain
cancer. Researchers are also looking at BNCT for treatment of lung and prostate cancer.
For this cancer treatment, researchers are utilizing epithermal (fast) neutrons, which come
from sources such as nuclear reactors and particle accelerators. However, since these are
fast neutrons they need to be slowed down for easy capture by boron-10. Shooting the neutron
beam through a hydrogen-rich moderator, will slow the neutrons down. If a tumor were deep
enough in the brain, the tissue between the skull and the tumor would function as the moderator,
allowing boron-10 to grab the neutron. Research has lead to a promising source of thermal
neutrons to irradiate human tissue with, but we need to get boron-10 into the body and
concentrate it specifically in the tumor cell. Researchers are trying to synthesize specific
boron-containing compounds that the cancer cells will preferentially absorb.
Since cancer cells grow faster than regular cells, they take up more amino acid than
regular cells. Several research groups are finding ways to incorporate boron atoms
into molecules that resemble amino acids.
For the time being however, all of this research and testing on how to effectively apply
boron neutron capture therapy into the medicine of tumor treatment is still in the
experimental stages. Researchers are making many new boron-containing compounds and
testing their efficacy in clinical studies. Hopefully in the near future BNCT will
prove effective in the fight against cancer.
And here are a few good links to get you started.
1. 2.
2. 3.
3.
This site is made possible by funding from the National Science Foundation (DUE-9981111).
|





