 |
 |
 |
 "What is Physics Good For?"
"What is Physics Good For?"
Extra credit is available at the end of this page. Please respond before 9 AM, Monday, October 9th, 2000.
In discussing electricity, we have had to discuss insulators. As is often the case, we have only refered to "generic" insulators: materials which do not allow charges to move. However, we have not discussed what real materials have this property. Perhaps the best known
In addition to being a good electrical insulator, glass has many other useful properties. It is a good thermal insulator (most material are either both or neither), and it is resistant to many corrosive chemicals. It is transparent, hard and easily colored; it is also easily formed into complex shapes. When broken, it makes an exceptionally sharp edge, and as a fiber it has high tensile strength. What is glass exactly? and why does it have these properties. A complete answer would require thousands of pages, and some questions are still under research, but we can cover some of the most important points right here.
The thing that really distinguishes glass is its lack of a crystal structure.
Considering that definition, it is really more correct to start talking about different kinds of glasses, rather than pretending that all glass is the same stuff. Many different chemical compositions can be made to form a disordered arrangement like the one shown above. Most of the common glasses that we are familiar with are mostly silicon dioxide often called "silica", the chemical formula is SiO2. However, very few glasses are pure silica. What gives glasses the properties mentioned above? In the familiar glasses (windows, bottles, etc.) the chemical bonding is enough to explain the insulating ability. To conduct electricity, a material must have "free" electrons which are not the same as excess electrons. To see the difference, let's consider a simple metal and a simple insulator. First lets do the metal; our sample contains 1000 atoms of sodium, which has an atomic number of 11. Therefore, when the piece has net charge 0 (no excess electrons) it has 11,000 electrons and 11,000 protons. Of those 11,000, about 10,000 are firmly bound to their respective nucleii, 10 electrons per nucleus. The other 1,000 are free to move about the sample. If the sample carries an electrical current, then these free electrons are the ones that move. In technical terms, the 10 fixed electrons per atom are called valence electrons and are said to be "localized." The single free electron is called a conduction electron and is said to be delocalized. Next, consider an insulator. A good example would be 1000 molecules of silicon dioxide (they could be arranged in a glassy structure or as a crystal of quartz). Silicon has atomic number 14, and oxygen 8 so this sample would contain 30,000 electrons. However, all of the electrons are localized; some are bound to a nucleus of a silicon or an oxygen atom, and some are shared in a chemical bond between two atoms. None of the electrons are delocalized, and thus the whole sample cannot carry current. In the 1970's physicsists discovered that it is possible to make metallic glasses (also called amorphous metals). Some of these materials have very unusual properties. For instance, some amorphous metals are almost as corrosion resistant as glass, and have extrordinary tensile strength (better than the best steels). Others have excellent magnetic properties. While most conduct electricity, they are poor conductors compared to crystalline metals. The reason? Although the amorphous metals have plenty of conduction electrons these electrons are constantly being scattered as they move through the maze of nucleii and valence electrons. Imagine trying to drive a car across a parking lot covered with large rocks. If the rocks are arranged in neat rows (like the atoms in a crystal) then it is easy to do. If the rocks are scattered around at random (like the atoms in a glass) then you have to go more slowly and make alot of turns.
 The key to making metals "glassy" is extremely rapid cooling, also known as "quenching". In the liquid state, the atoms of any material are moving around in a disordered way. If the material cools slowly, then atoms have time to "find" each other and form chemical bonds in an orderly way. If the material is quenched rapidly, then the atoms do not have time to sort themselves into an orderly arrangement. They simply stop where they are, and form whatever bonds they can. Thus, they become trapped in the disordered arrangement typical of the liquid state.
You can get a lot more information about this subject on the internet. Here are a few search enginesAnd here are a few good links to get you started.
Research Questions (1 point extra credit each!)
This site is made possible by funding from the National Science Foundation (DUE-9981111). |
 material that has excellent insulating properties is glass. Early in the history of the electric power industry, most of the insulators used were made of glass. Since the insulating properties of the glass did not depend on color, these insulators were often made of whatever color glass was around at the time. As a result, many were brightly colored and some were
material that has excellent insulating properties is glass. Early in the history of the electric power industry, most of the insulators used were made of glass. Since the insulating properties of the glass did not depend on color, these insulators were often made of whatever color glass was around at the time. As a result, many were brightly colored and some were 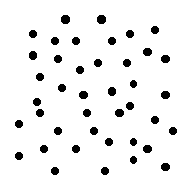 Most familiar materials are made up of atoms in an orderly arrangement. The details of this arrangement are quite simple for some materials, for instance, iron, salt and diamond (carbon). Other materials may have extremely complex structures.
Most familiar materials are made up of atoms in an orderly arrangement. The details of this arrangement are quite simple for some materials, for instance, iron, salt and diamond (carbon). Other materials may have extremely complex structures.
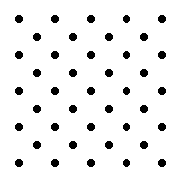 Because of their lack of crystal structure, glasses are sometimes called amorphous materials.
Because of their lack of crystal structure, glasses are sometimes called amorphous materials.
 History of Glass
History of Glass