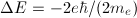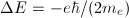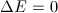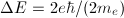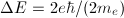The 2p state level in hydrogen is 6-fold degenerate when the spin is taken into account (3 possible values for m and 2 possible values for ms). On the other hand, in the presence of a magnetic field, the anomalous Zeeman effect splits this level into five energy levels: 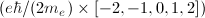 . That means that one of those five levels must be degenerate. Which is it?
. That means that one of those five levels must be degenerate. Which is it?
-
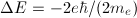
-
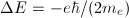
-
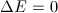
-
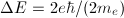
-
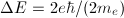
A hydrogen atom is placed in a magnetic field. How many energy levels do the normally degenerate 4f states split into?
- 1
- 3
- 4
- 8
- 9
Which of the following electronic configurations are possible?
- 3 1s electrons, two with spin up and one with spin down
- 2 1p electrons, one with spin up and one with spin down
- 4 2p electrons, two with spin up and two with spin down
- 4 2p electrons, all with spin up
- 2 3s electrons, one with spin up and one with spin down
- 5 3d electrons, all with spin up
- one 1s and one 2s electron, both with spin up
A classical magnetic moment is placed in a magnetic field which makes a nonzero angle of θ with it. What will the subsquent motion of the magnetic moment be in the absence of any other forces?
- Its orientation will oscillate back and forth.
- Its position will oscillate back and forth.
- It will align itself with the magnetic field.
- It will accelerate in the direction of the magnetic field.
- It won't move.
A stationary electron is put in a uniform magnetic field. Describe its subsequent motion.
- Nothing will change.
- Its position will oscillate back and forth.
- Its orientation will oscillate back and forth.
- It will accelerate in the direction of the magnetic field.
- It will align itself with the magnetic field.
In the Stern-Gerlach experiment, a beam of spin-1/2 silver atoms passed through a non-uniform magnetic field. Because of the spin of the atoms and the fact that the potential energy changed with position, there was a net force on the silver atoms which was in one direction for spin-up atoms and the opposite direction for spin-down atoms. What do you think Stern and Gerlach saw when they ran this experiment?
- The original beam split into two distinct beams, one for spin-up and one for spin-down.
- The original beam wasn't spread out since the average spin was zero.
- The orignal beam was spread out in a broad direction for each continuous possible orientation of the spin.
- The original beam was spread into three peaks: spin up, spin down, and spin zero.
- The beam didn't spread out because no "measurement" was made.
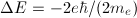
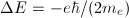
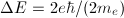
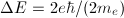
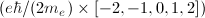 . That means that one of those five levels must be degenerate. Which is it?
. That means that one of those five levels must be degenerate. Which is it?