Shapes
of Molecules
This
is an archival version of the web assignment.
It is after the due date, so the assignment
can no longer be submitted.
The purpose of this assignment is to
examine some molecules and explore the geometry and
bond angles around the atoms. Remember, that the first
step is to draw the Lewis dot structure of the molecule
making sure that all the valence electrons present in
the molecule are shown in the drawing. Once the Lewis
dot structure has been correctly drawn, find the atom
whose geometry you want to describe. Determine the number
of electron charge clouds (atoms and/or lone pairs)
around this atom to predict the bond angle.
Now look to see how the atoms are arranged
to predict the shape. See chapter 3 in the Denniston
text for more information.
Let's look at the structure
of aspartic acid, one of the amino acids, as an example.
The correct Lewis structure has already been drawn for
you.
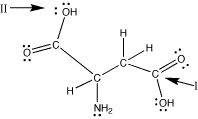
aspartic acid |
Look at the carbon
indicated by arrow I. There are 3 electron charge
clouds around it: a single bond to oxygen, a
double bond to oxygen, and a single bond to
carbon. The farthest apart 3 "things"
can be from each other is a trigonal planar
arrangement with the charge clouds 120oapart.
Thus, the O-C-O bond angle is 120o.
As all of the charge clouds are connected to
atoms, the geometry around this carbon atom
is trigonal planar.
Now look at the oxygen indicated
by arrow II. There are 4 electron charge clouds
around it: a single bond to carbon, a single
bond to hydrogen, and two lone pairs of electrons.
The farthest apart 4 "things" can
be is in a tetrahedral arrangement where the
charge clouds are approximately 109o
apart. Thus, the C-O-H bond angle here is about
109o. The geometry around this oxygen
is bent. (We have to think tetrahedral
for the arrangement of the electron charge clouds
to determine the bond angles. To describe the
shape around this oxygen, we look at the atoms.
Since only two of the charge clouds are atoms,
the shape around this oxygen atom is described
as bent.) |
Now it is your turn. Look
at the Lewis dot structure of caffeine. Determine the
bond angles and geometry around atoms indicated by the
arrows.
| 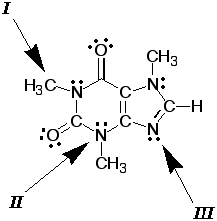
caffeine |
| 10. |
Arrows II &
III both point to nitrogen atoms. Explain
in detail why your answers to 4-6
were different from your answers to 7-9. |
| |
|
|
Here is a ball and stick
picture of PFCl4. Phosphorus is shown in
blue and is the central atom. The fluorine atom is shown
in green and the chlorine atoms are shown in red. Notice
that three of the chlorine atoms are in the same plane
and the plane also includes the phosphorus atom.
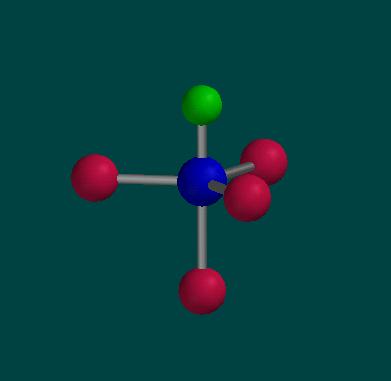
PFCl4 |
|
This is
an archival version of the web assignment. It is
after the due date, so the assignment can no longer
be submitted.
